1. Product Overview of Rveiling® HA-CaHA Injectable Facial Filler
What is HA-CaHA Injectable Facial Filler?
Milky White Biodegradable Gel Implant
Rveiling® HA-CaHA Injectable Facial Filler is a sterile, milky white, biodegradable viscous gel implant designed for professional use in B2B aesthetic applications. Packaged in pre-filled syringes (1 mL, 2 mL, 3 mL), it provides both instant volumizing effects and long-lasting tissue restoration.
Key Benefits for Facial Rejuvenation
Immediate Volume and Long-Lasting Effects
The filler combines the hydrating properties of sodium hyaluronate with calcium hydroxyapatite microspheres, achieving immediate facial volume restoration while promoting collagen synthesis for long-term rejuvenation. Ideal for cheek augmentation, wrinkle correction, and facial contouring.
2. Structure and Composition
Active Ingredients
High-Quality Biocompatible Components
Sodium Hyaluronate (20 mg/mL) – Provides hydration and immediate tissue filling.
Calcium Hydroxyapatite Microspheres (30.7%) – Forms a scaffold for collagen regeneration and long-lasting contour.
Lidocaine Hydrochloride (0.3%) – Reduces injection discomfort.
Sodium Chloride & Phosphate Buffer System – Maintains physiological pH and biocompatibility.
Sterile Injection Water – Ensures a stable gel consistency.
Gel Carrier and Microsphere Interaction
Synergistic Mechanism for Optimal Results
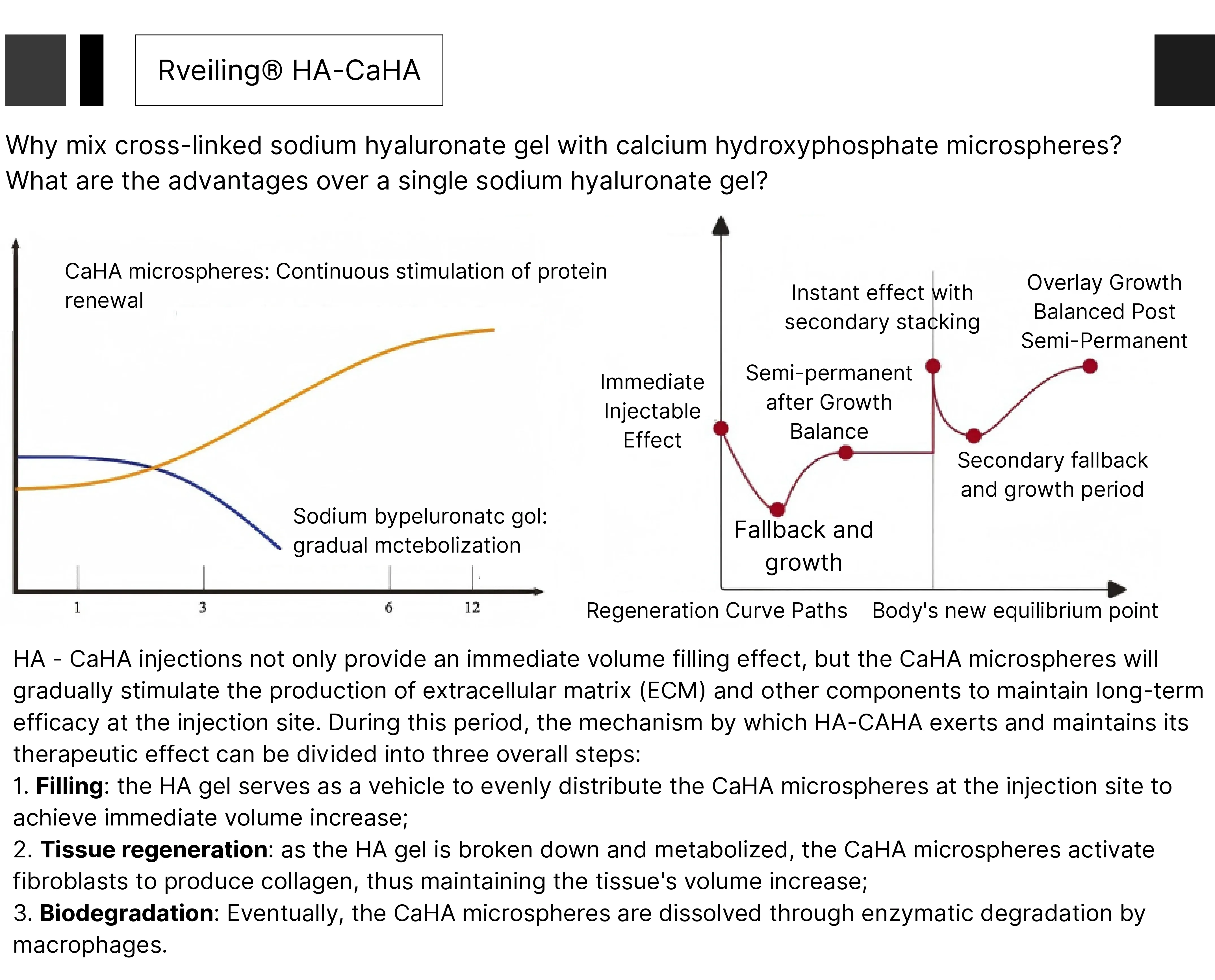
The cross-linked sodium hyaluronate gel provides immediate filling, while CaHA microspheres remain at the injection site to stimulate collagen production, creating a natural-looking, long-lasting rejuvenation effect.
3. Product Performance
Particle Size and Injection Precision
Safe and Effective Dermal Filler
Rveiling® HA-CaHA microspheres have a median particle size of 32 μm ± 6 μm, ensuring smooth injection and uniform volume restoration. 95% of microspheres measure between 25–50 μm, optimized for deep dermis, subcutaneous tissue, and periosteum injections.
Mechanism of Action
From Physical Filling to Biological Regeneration
Immediate volume from the gel carrier.
Collagen induction around stable CaHA microspheres.
Activation of fibroblasts and macrophages for Type I and III collagen synthesis.
Gradual microsphere biodegradation while maintaining structural support.
4. Indications and Applications
Facial Correction and Contouring
Comprehensive Rejuvenation Solutions
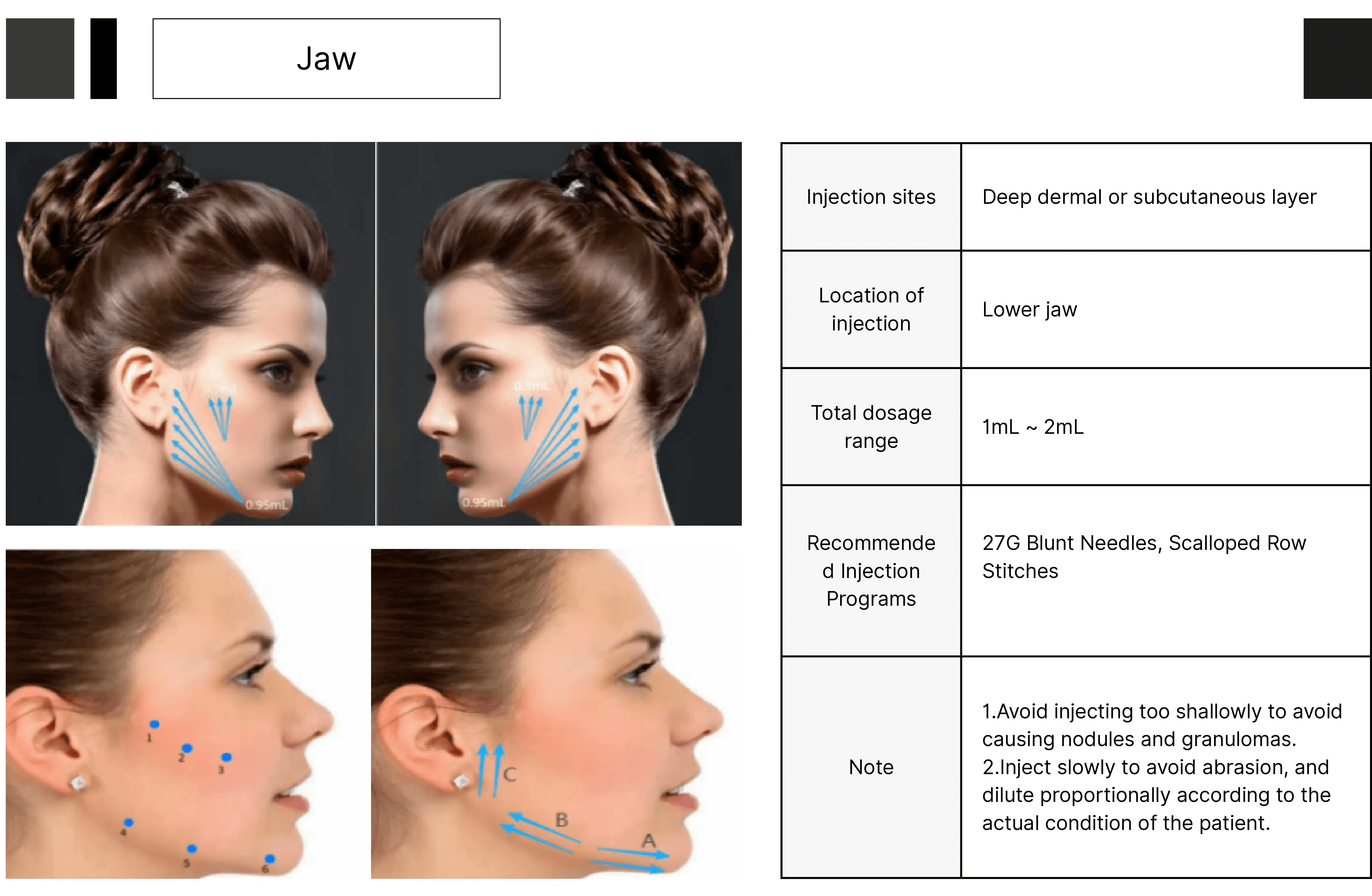
Ideal for correcting deep facial depressions, marionette lines, jawline contouring, and cheek augmentation. Also suitable for facial fat loss restoration in patients requiring specialized care.
Multi-Area Treatment Options
Wrinkle Repair Across the Body
HA-CaHA filler can be diluted for treatments on the face, neck, arms, abdomen, buttocks, and other areas requiring volume restoration and skin rejuvenation.
5. Usage Guidelines and Safety
Sterilization and Single-Use Precautions
Ensuring Safe Clinical Application
The filler syringe is sterilized via high-temperature steam, and the injection needle uses ethylene oxide sterilization. Strict sterile surgical procedures are mandatory to maintain safety and prevent contamination.
Comfortable Injection Experience
Lidocaine for Local Anesthesia
The inclusion of 0.3% lidocaine hydrochloride ensures minimal patient discomfort during injection, promoting better tolerance and satisfaction.
6. Product Advantages
Long-Lasting but Biodegradable
Natural Results Without Permanent Implants
CaHA microspheres provide durable facial contour enhancement while gradually absorbing and integrating with the body’s natural tissue over time.
Professional-Grade Quality
Latex-Free, Pyrogen-Free, Biocompatible
Rveiling® HA-CaHA filler is manufactured to strict quality standards, ensuring safe, predictable, and repeatable results for professional aesthetic clinics.
Product Specifications
Syringe Volume Options
Flexible Dosing for Different Treatments
Available in 1 mL, 2 mL, and 3 mL pre-filled syringes to meet diverse clinical requirements.
Shelf Life
36-Month Stability
Store in a cool, dry environment. Single-use only for consistent clinical outcomes.
Products Status

Mechanism of action

Rveiling® HA-CaHA Composite Filler: Dual-action in one syringe. Deeply builds firm structural support to lift facial contours; finely sculpts superficial layers to create natural shaping power for plump, dimensional skin. Simultaneously stimulates collagen regeneration for long-lasting firmness and skin texture improvement.
Reveiling® HA-CaHA facial filler, when implanted into soft tissue, undergoes gradual degradation where the gel matrix breaks down before the CaHA microspheres. These microspheres provide long-term structural support while stimulating natural collagen regeneration.

Revealing® HA-CaHA Implant Tissue: Pre- and Post-Treatment Effect Comparison Long-lasting activation of collagen regeneration Stabilizes facial tissue volume Reshapes smooth contour lines Achieves a natural, youthful facial appearance.
Back of the hand

Key attributes
Transport conditions | transported at normal temperature, the transport cycle should not exceed1 month |
|---|---|
Storage condition | 2-25°C,prohibit freezing, avoid sunlight |
Specification | 1 mL/syringe 2 mL/syringe 3 mL/syringe |
Transport Pakeage | 50-100 pieces per box |
HA Gel Carriers | 69.0% |
CaHA Microsphere | 30.7% |
Lidocaine | 0.3% |
Injection Areas |
It should be used by an authorized practitioner. Do not re-sterilize or mix with other products. |
Injection Depth | Subcutaneous tissue, from the middle to deep layers; periosteum, upper layer |
Duration | 12-24 Months |
Before treatment:
Tell your physician about all medications you are taking and your past treatment history.
Foods that increase the chance of bleeding and cause bruising should be avoided for the time being, such as: ginkgo, fish oil, aspirin drugs, vitamin E, etc.
Consult your physician if you are allergic to the drug, breastfeeding, or during pregnancy.
After treatment :
Avoid alcohol and strenuous exercise for twenty-four hours.
Apply light make-up after twenty-four hours and avoid the location of the pinholes.
Intrusive treatments, facial massages, and facial aesthetic procedures are not recommended for two weeks.
Avoid prolonged sun exposure, exposure to UV light, cold temperatures, and hot treatments.
Temporary redness, swelling, soreness and bruising are natural and will subside in five to seven days.
FAQs
1. Injectable Hydroxyapatite Microsphere Facial Filler
Our calcium hydroxyapatite injectable facial filler is formulated with biocompatible calcium hydroxyapatite microspheres suspended in a biodegradable gel particle carrier. Following implantation, the gel particles gradually degrade and metabolize, while the microspheres provide long-term structural support and stimulate natural collagen regeneration.
2. Can we customize packaging for OEM or private label production?
Yes. As a professional B2B manufacturer and supplier, we offer OEM and ODM customization services, including logo and packaging design. We support clinics, distributors, and medical aesthetic brands with flexible collaboration models.
3. What are the primary applications of injectable calcium hydroxylapatite facial fillers?
This injectable implant filler is suitable for correcting moderate to severe facial wrinkles, contouring, wrinkle reduction, skin folds, and restoring volume loss in the hands. Injected into the deep dermis or upper soft tissue above the periosteum, it delivers non-permanent yet long-lasting enhancement effects.
4. How long do the effects of hydroxyapatite microsphere facial fillers last?
Typically, results persist for 24 to 36 months or longer, depending on the injection site, technique, and patient metabolism. The calcium hydroxyapatite microspheres continue stimulating collagen growth, delivering sustained volume and firmness over time.
5. What certifications and quality standards do you provide for B2B partners?
Our manufacturing strictly adheres to ISO 13485, GMP, and CE certification—globally recognized standards for medical device production quality and safety. Each production batch undergoes exhaustive biological performance testing covering biocompatibility, sterility, pyrogen-free status, and functional integrity. This rigorous process ensures 100% batch compliance, delivering consistent reliability you can trust.












