Product Overview: Rveiling® Cross-Linked Sodium Hyaluronate Facial Filler
1. Product Positioning & Intended Use
Professional Injectable Dermal Filler Raw Material
Rveiling® Cross-Linked Sodium Hyaluronate Gel for Injection is a medical-grade injectable gel designed for professional aesthetic applications. As a manufacturer-oriented solution, it is developed to support dermal filler production, OEM/ODM cooperation, and clinical aesthetic use under regulated medical environments.
The product is supplied in sterile, pre-filled syringes and formulated to meet the performance, safety, and consistency requirements expected by global aesthetic medicine brands and professional distributors.
2. Available Models and Specifications
Syringe Volume and Injection Depth Options
Cross-Linked Sodium Hyaluronate Gel for Injection is available in standardized syringe formats to accommodate different clinical protocols and product development needs:
1 mL / syringe
2 mL / syringe
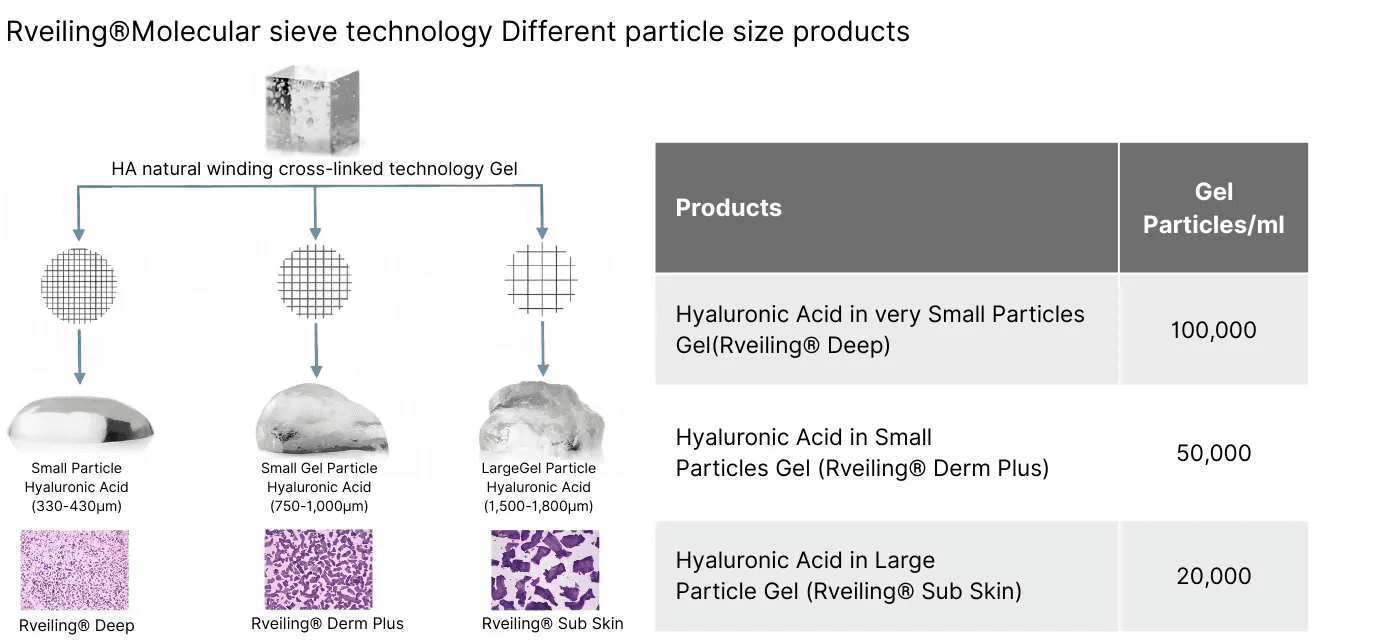
Injection depth models include:
Derm: suitable for mid-dermal correction
Deep: designed for mid-to-deep dermal tissue applications
Product Composition and Structural Characteristics
1. Ingredient Profile
Precisely Balanced Injectable Formulation
The formulation is composed of the following pharmaceutical-grade components:
Sodium hyaluronate
Lidocaine hydrochloride
BDDE cross-linking agent
Sodium chloride
Phosphate buffer system
Water for injection
Each ingredient is selected to ensure stability, injectability, and biocompatibility while supporting consistent gel performance.
2. Structural Composition
Cross-Linked Gel Particle Suspension
The product consists of a suspension of cross-linked sodium hyaluronate gel particles combined with a controlled proportion of free sodium hyaluronate. The hyaluronic acid is produced via microbial fermentation, ensuring high purity and reproducibility.
The gel is packaged in a single-use, pre-filled syringe to minimize contamination risk and ensure precise dosing. Lidocaine hydrochloride is incorporated to reduce injection discomfort and improve patient experience during treatment.
Mechanism of Action and Clinical Performance
1. Mechanism of Action
Dermal Tissue Filling and Volume Restoration
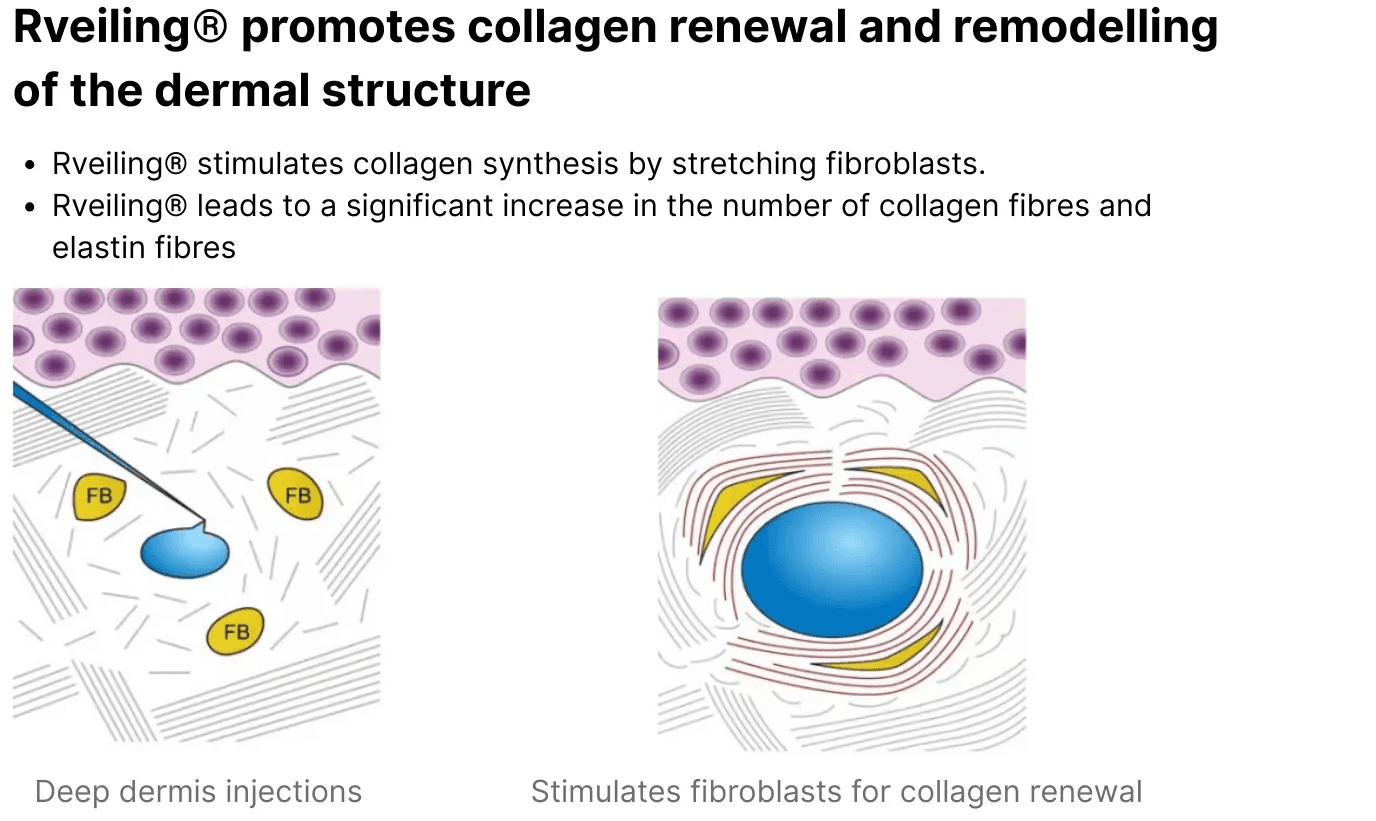
Cross-Linked Sodium Hyaluronate Filler functions as a dermal tissue filler. Upon injection into targeted dermal layers, the gel provides mechanical support, restores volume, and enhances contour definition.
The cross-linked HA matrix maintains structural integrity within the tissue, contributing to sustained aesthetic outcomes.
2. Indications for Use
Mid-to-Deep Dermal Correction Applications
This product is indicated for injection into the mid-to-deep layers of facial dermal tissue in adults aged 18 years and above. Typical application areas include:
Mid-face wrinkles
Deep dermal depressions
Chin contouring
Cheek volumization
Jawline definition
The treatment aims to improve facial appearance by correcting shape and restoring youthful contours.
EYE-CATCHING RESULTS
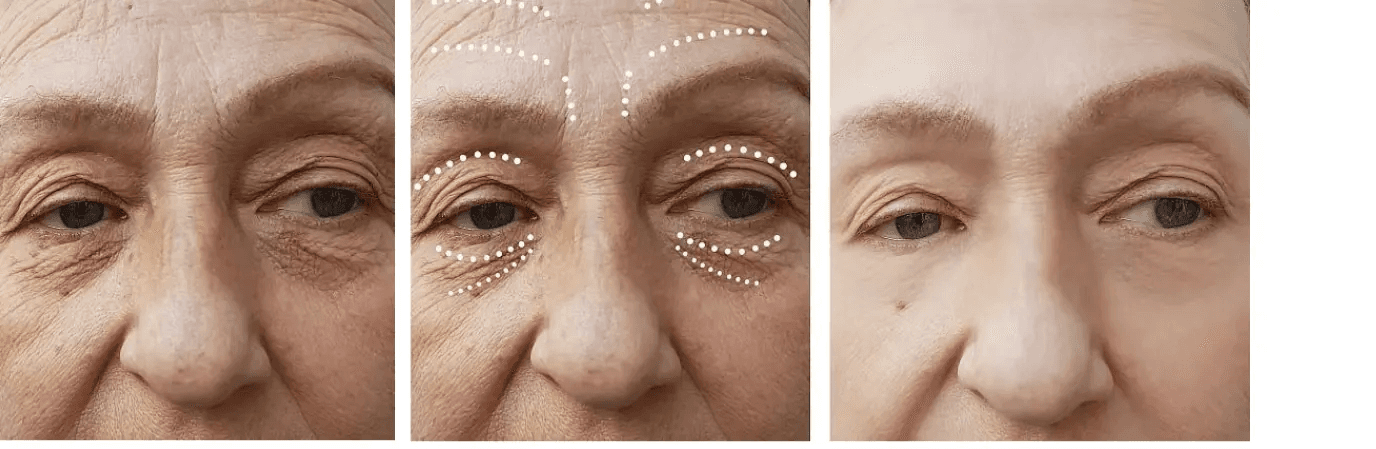
Cross-linked Sodium Hyaluronate Gel Granules for Injection
Key attributes
Ingredients | Sodium Hyaluronate, Lidocaine Hydrochloride, BDDE crosslinking agent, Sodium Chloride, Injection Water Phosphate Buffer System |
|---|---|
Product Specifications | 1mL/syringe, 2mL/syringe |
Model | Derm/Deep |
Injection Force | 10-40N |
Gel Consistency | Viscoelastic |
Indications | Correction of skin depressions, facial contouring |
Injection Depth | Mid to deep dermis |
Injection Areas | For treating facial wrinkles and deep skin depressions, including the chin, cheeks, jawline, and temples. |
Recommended Needle | 27G sharp needle/25G blunt cannula needle |
Recommended Treatment Protocol | Treatment plans should be individualized. A qualified practitioner should select injection strategies targeting the mid-dermis or deep dermis based on skin thickness and muscle activity characteristics. |
Duration of Effect | Results typically last 12-18 months. Effectiveness and duration depend on defect type, injection depth, and individual physiology. |
Professional Administration | This product must be administered by authorized professionals in regulated medical facilities. |
Recovery Time | Mild redness and swelling may persist for 1-2 days post-injection, with full recovery typically occurring within 3-7 days. |
FAQs
1. Why does cross-linked hyaluronic acid last longer than non-crosslinked HA?
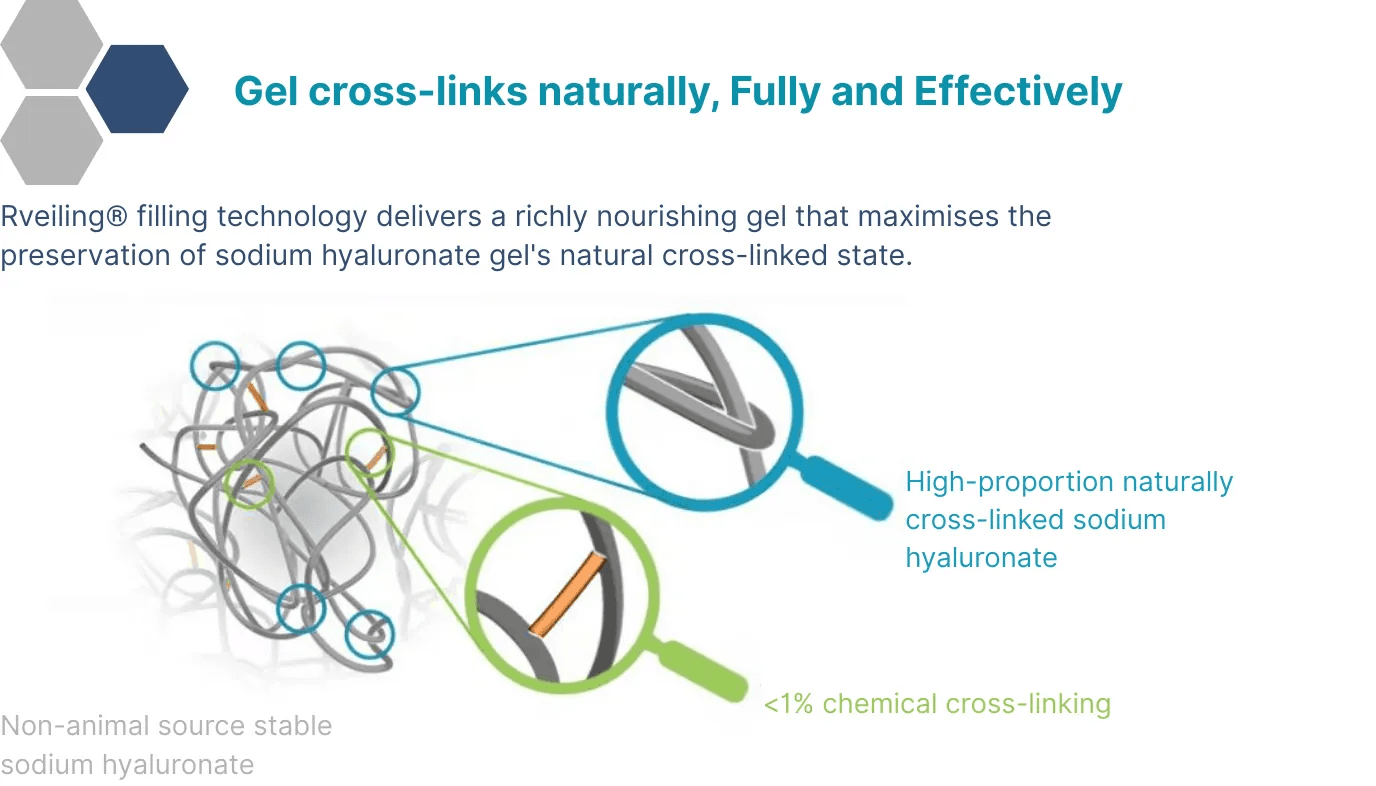
Cross-linked hyaluronic acid possesses a stable three-dimensional structure that resists natural enzymatic degradation within the skin. While non-cross-linked hyaluronic acid breaks down within days to a week, cross-linked hyaluronic acid persists in the body for months or even longer, delivering extended filling and hydration effects. Rveiling® employs natural HA entanglement cross-linking technology combined with an efficient BDDE-modified cross-linking process, ensuring excellent biocompatibility and long-lasting efficacy in product applications.
2. Is your cross-linked hyaluronic acid derived from animals?
No. Our sodium hyaluronate raw material is produced through microbial fermentation, utilizing non-animal-derived ingredients. This reduces the risk of allergic reactions and ensures the implantable filler maintains a high level of safety throughout the treatment process.
3. What is the purpose of lidocaine in the injectable gel?
Lidocaine (0.3%) is used to reduce discomfort during injection, alleviate local pain, and enhance the patient's treatment experience. Each batch of our product undergoes rigorous quality control to ensure the safety of lidocaine concentration in clinical use.
4. How does your cross-linking process preserve the natural structure of hyaluronic acid?
Our cross-linking process employs a dual approach: natural entanglement cross-linking technology for high molecular weight HA combined with low-level BDDE chemical modification cross-linking. This stabilizes the molecular structure of sodium hyaluronate while preserving its natural biological architecture. This balanced outcome delivers exceptional viscoelasticity, biocompatibility, and water retention properties during therapeutic enhancement.
5. How can clinics or distributors work with your factory for OEM or bulk supply?
We are a specialized manufacturer and global supplier dedicated to partnering with medical clinics, brands, and distributors for OEM, private label, or bulk supply. We provide comprehensive technical documentation, quality certifications, and global logistics support to ensure safe and efficient delivery.













