PLLA-PEG Injectable Facial Filler Overview
Rveiling® PLLA-PEG Injectable Facial Filler is a milky white, sterile, biodegradable viscous gel implant. Utilizing a pre-filled syringe system, it combines instant filling with long-lasting effects to restore skin tissue volume through injection, achieving facial rejuvenation and aesthetic contouring.
1. Product Specifications
This sterile, white lyophilized powder features a porous sponge-like microstructure for superior storage and transportation. Reconstitute with Sterile Water for Injection (SWFI) to yield a uniform, milky-white suspension of stably dispersed PLLA-PEG microspheres, with dilution ratios tailored to filling or regenerative needs in targeted areas.
Vial and Composition Details
Sizes: 220mg/vial or 360mg/vial for precise, single-use dosing.
Ingredients: PLLA-PEG copolymer microspheres, mannitol, sodium hyaluronate.
Stability: 24-month shelf life through irradiation sterilization.
Adjustable concentrations support versatile applications across cheeks, temples, forehead, and hands.
Mechanism of Action
Rveiling® shifts from conventional filling to regeneration by positioning PLLA-PEG microspheres as a temporary scaffold. Injection prompts a controlled, non-inflammatory response, recruiting macrophages and fibroblasts that synthesize Type I and III collagen around the microspheres, building an autologous extracellular matrix.
1. Regeneration Timeline
Microspheres trigger repair signals and fibroblast attachment.
Collagen deposition and cross-linking form permanent tissue support.
Material hydrolyzes into lactic acid, CO2, and water, fully metabolizing without residue.
This yields progressive volume maintenance, enhanced elasticity, and improved texture over 24 months.
Clinical Applications
Indicated for adults 18+ combating soft tissue volume loss and facial laxity. Delivers dual-action rejuvenation—immediate lift plus neocollagenesis—for natural autologous reconstruction in multiple zones.
1. Patient Suitability
Ideal for anti-aging seekers desiring firmness and contour enhancement.
Requires physician review for allergies, keloids, pregnancy, or breastfeeding.
Core Technology Edge
The hyaluronate carrier system ensures even microsphere distribution, exact dosing from pre-filled vials, and inflammation reduction via high biocompatibility. This innovation powers safer, more predictable PLLA-PEG injectable filler outcomes for clinical excellence.
B2B Factory Solutions
Collaborate with our Poly-L-Lactic Acid-Polyethylene Glycol facility for bulk PLLA-PEG microspheres production, custom formulations, and white-label packaging. Access competitive pricing, rapid scaling, and global supply chain support to dominate the facial implant filler market.

Rveiling® PLLA-PEG filler microspheres continuously stimulate fibroblasts to induce the secretion and synthesis of collagen in the human body. Consequently, they are widely used in medical aesthetic fillers and tissue regeneration and repair therapies.

Rveiling® PLLA-PEG Microspheres Continuously Induce Human Fibroblasts to Secret and Synthesize Collagen

Rveiling® PLLA-PEG Microspheres Appearance: Smooth spherical PLLA-PEG microsphere particles, non-toxic, extremely safe, non-irritating, uniform particle size, and comparative biological response effects upon implantation into soft tissue.

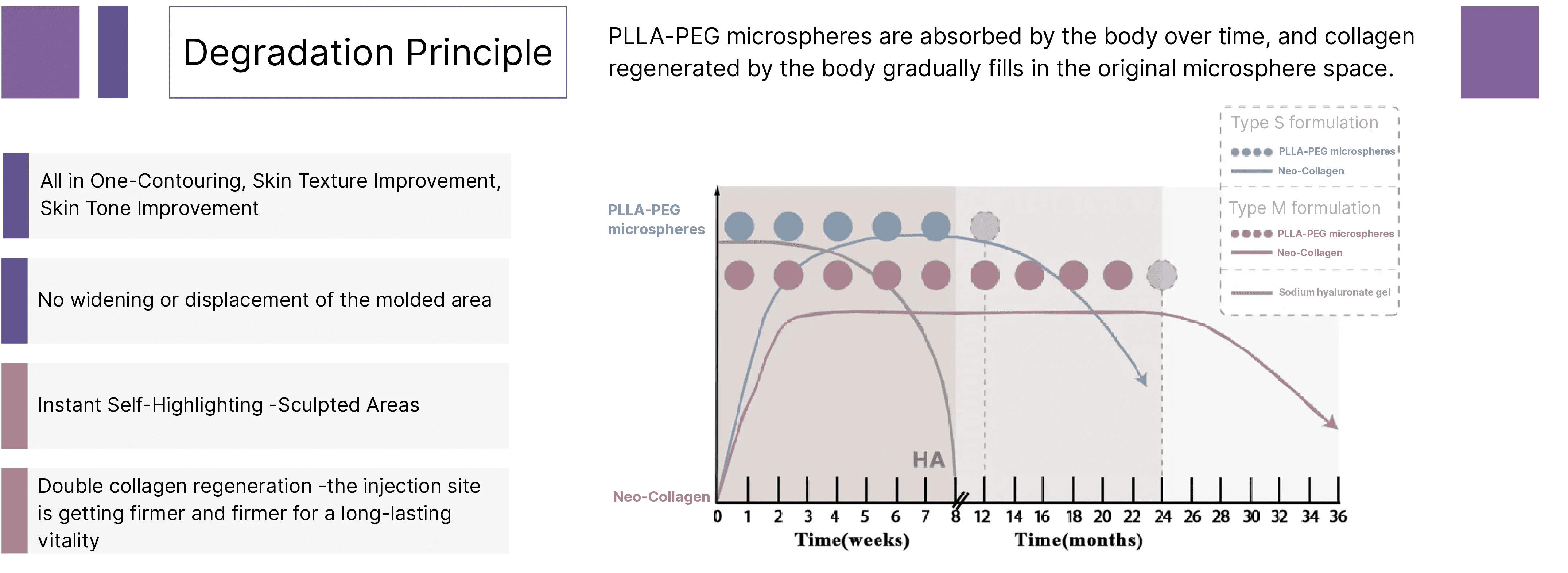
Rveiling® PLLA-PEG microspheres undergo gradual degradation within tissue, stimulating the progressive regeneration of endogenous collagen. As new collagen replaces the original space occupied by the microspheres, it achieves natural filling and structural support effects.
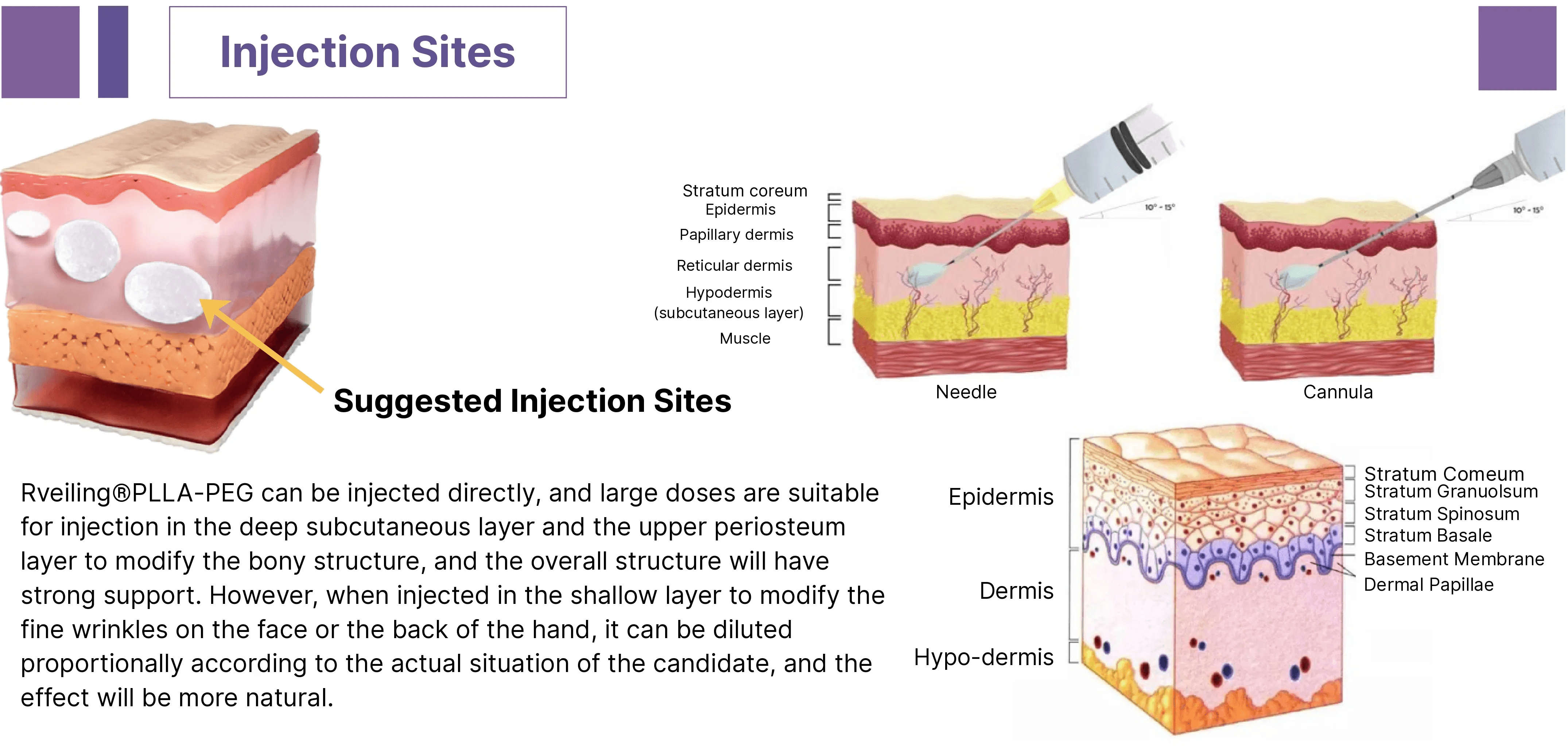
Rveiling® PLLA-PEG Facial Filler delivers deep structural support or natural, three-dimensional contouring effects depending on the injection layer.
Poly-L-Lactic Acid-Polyethylene Glycol Product Status

Key attributes
Specification | 1 mL/syringe, 2 mL/syringe, 3 mL/syringe |
|---|---|
Storage condition | 2-10℃, prohibit freezing, avoid sunlight. |
Transport conditions | Transported at normal temperature, the transport cycle should not exceed1 month. |
Usage Restriction | Do not re-use |
Sterilization Method | High-temperature steam sterilization |
Usage Warning | Do not use if packaging is damaged |
Sterilization Restriction | Do not resterilize |
Cross-linked Sodium Hyaluronate | 16 mg/mL |
Poly(L-lactic acid)-poly(ethylene glycol) microspheres | 20.7 % |
Lidocaine Hydrochloride | 3 mg/mL |
FAQs
Rveiling® PLLA-PEG Injectable Facial Filler
Rveiling® PLLA-PEG Injectable Facial Filler utilizes PLLA-PEG material with excellent biocompatibility to produce microspheres of uniform particle size. PLLA-PEG is an amphiphilic block copolymer primarily applied in biodegradable medical fields. Its hydrophilic outer layer enhances cell adhesion and tissue compatibility. The hydrophobic PLLA segments aggregate to form the core, while the hydrophilic PEG segments extend outward to create the shell. The microspheres are suspended within a gel particle carrier. Following injection and implantation, the gel particles gradually degrade, while the microspheres provide long-term structural support and continuously induce human fibroblasts to secrete and synthesize collagen. Over time, PLLA-PEG undergoes gradual biodegradation.
2. Can outer packaging be customized for OEM or private label production?
Yes. As a professional B2B manufacturer and supplier, we offer OEM and ODM customization services, including logo and packaging design. We support clinics, distributors, and medical aesthetic brands with flexible collaboration models.
3. What are the primary applications of Rveiling® injectable PLLA-PEG facial filler?
Rveiling® PLLA-PEG injectable implant filler is an off-white, viscous, sterile, latex-free, pyrogen-free, semi-solid gel, biodegradable dermal implant filler with immediate results. This implant filler is indicated for correcting facial wrinkles and deep dermal depressions, cheek augmentation, marionette lines, jawline contouring, and restoring/correcting facial fat loss (atrophy) in AIDS patients. Injected into the soft tissue above the periosteum, it provides non-permanent yet long-lasting enhancement.
4. How long do the effects of Rveiling® PLLA-PEG facial filler last?
Typically, results persist for 12 to 24 months, depending on the injection area, the clinician's technique, and the patient's metabolism. The sodium hyaluronate gel carrier delivers immediate filling effects. The gel particles degrade before the microspheres, while the PLLA-PEG microspheres continue to stimulate human fibroblasts to secrete and synthesize collagen, providing long-lasting volume and firmness over time.
5. What certifications and quality standards do you offer for B2B partners?
Our manufacturing strictly adheres to ISO 13485, GMP, and CE certification—globally recognized standards for medical device production quality and safety. Each production batch undergoes rigorous full-performance biological testing, covering biocompatibility, sterility, and all product performance metrics. This stringent process ensures 100% batch compliance, delivering consistent reliability you can trust.












