1. Product Overview – Rveiling® Cross-linked Sodium Hyaluronate Gel
Professional Cross Linked Hyaluronic Acid Dermal Filler
Designed for Structural Support and Natural Volume Restoration
Rveiling® + Lidocaine Cross-linked Sodium Hyaluronate Gel for Injection is a medical-grade cross linked hyaluronic acid dermal filler developed for professional aesthetic and reconstructive applications. Supplied in a 20 mL pre-filled syringe, this injectable HA gel delivers high volumizing capacity, strong tissue support, and seamless integration, making it suitable for deep facial and subcutaneous augmentation.
Product Specifications
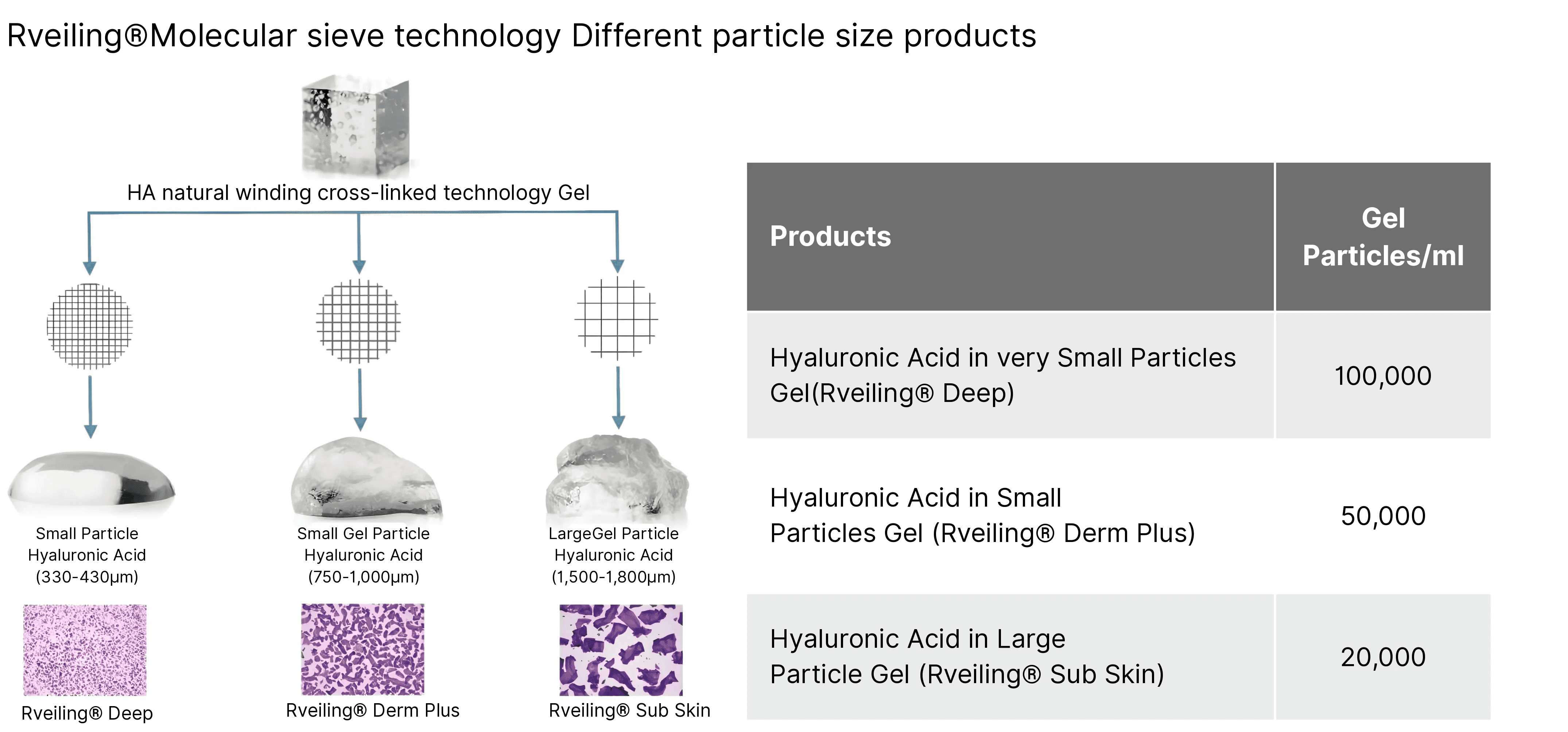
Large-Volume Syringe for Professional Use
Specification: 20 mL / syringe
Model Options: Derm Plus / Sub Skin
Application Layer: Deep subcutaneous layer and superficial periosteal layer
Shelf Life: 36 months
This configuration supports clinics and distributors seeking long-lasting cross linked hyaluronic acid injectable fillers for advanced contouring and volume restoration.
2. Composition and Ingredients
High-Purity Active Ingredients
Balanced Formula for Safety and Performance
Sodium Hyaluronate: 25 mg/mL
Lidocaine Hydrochloride: 3 mg/mL
BDDE (Cross-linking Agent)
Sodium Chloride
Water for Injection (WFI)
Phosphate Buffer System
This formulation ensures high viscosity, strong cohesion, and predictable degradation, aligning with professional requirements for cross linked hyaluronic acid dermal filler manufacturers.
3. Mechanism of Action
Advanced Cross-Linking Technology
Homogeneous HA Gel with High Elastic Modulus
Rveiling® employs high molecular weight hyaluronic acid natural entanglement cross-linking combined with chemical modification. This patented technology creates a tightly cross-linked HA network, resulting in a homogeneous gel structure with an optimal balance between elastic modulus (G’) and cohesive force.
Natural Tissue Integration
eamless Appearance and Long-Lasting Support
The cross linked hyaluronic acid gel integrates smoothly into surrounding tissues, providing deep structural support while maintaining natural facial movement. This makes it suitable for facial contouring, lifting, and volume augmentation with a soft, natural finish.
4. Indications and Treatment Applications
Indicated Patient Population
Adults Requiring Tissue Volume Augmentation
Rveiling® + Lidocaine Cross-linked Sodium Hyaluronate Gel is indicated for individuals aged 18 years and older requiring tissue volume enhancement and structural support.
Treatment Areas
Deep Structural and Contour Correction
Facial volume augmentation
Structural lifting support
Shape correction
Contour enhancement
Deep tissue support over periosteum
This product is particularly suitable for professionals seeking cross linked hyaluronic acid filler for facial volumization and contouring.
5. Core Technology Advantages
Patented Cross-Linking Process
Enhanced Stability and Prolonged Degradation
The novel cross-linking technology achieves tighter molecular bonding within the HA structure, improving:
Biocompatibility
Resistance to enzymatic degradation
Longevity of aesthetic results
This ensures long-lasting cross linked hyaluronic acid dermal filler performance without compromising safety.
Optimal Adhesion and Support
Reliable Performance in Deep Tissue Layers
The gel’s cohesive properties allow it to maintain shape and position post-injection, delivering reliable lifting and support even in high-movement or high-stress areas.
6. Safety, Sterilization, and Usage
Sterilization Method
Medical-Grade Sterility Assurance
Gel sterilized by high-temperature steam
Pre-filled syringe format
Single-use only to prevent cross-contamination
Lidocaine-Enhanced Comfort
Improved Patient Tolerance
The inclusion of 3 mg/mL lidocaine hydrochloride helps minimize injection discomfort, supporting smoother procedures and higher patient satisfaction.
7. Quality Management and Manufacturing Standards
Certified Production System
CE and ISO 13485 Compliance
Manufactured under a standardized quality management system, Rveiling® adheres strictly to CE and ISO 13485 requirements. Every stage—from raw material inspection to finished product release—is documented and traceable.
Batch Consistency and Reliability
Trusted B2B Supply Capability
This ensures consistent gel performance across batches, meeting the expectations of global distributors, clinics, and private-label partners seeking a reliable cross linked dermal filler manufacturer.
Key attributes
Ingredients | Sodium Hyaluronate, Lidocaine Hydrochloride, BDDE, Sodium Chloride, Water for Injection (WFI), Phosphate Buffer System |
|---|---|
Model | Derm Plus/Sub Skin |
Product Specification | 20mL/syringe |
Gel Hardness | Viscoelastic |
Indications | Provides structural support, lifting, and contour correction to improve skin appearance. |
Injection Depth | Deep subcutaneous, supraperiosteal |
Recommended Needle | Blunt-tip cannula |
Treatment Areas | Chest, buttocks, or large-area depressions requiring structural support, lifting, and contour correction. |
Rveiling®+ Lidocaine Cross-linked Sodium Hyaluronate Gel for Injection begins with the “natural entanglement” of high-molecular-weight hyaluronic acid (HA) chains, forming an initial three-dimensional network. This is followed by precise anchoring and reinforcement through “chemical modification,” constructing a stable gel molecular structure that combines flexibility with strong support. This delivers a longer-lasting filling effect.
FAQs
1. Why does cross-linked hyaluronic acid last longer than non-cross-linked hyaluronic acid?
Cross-linked Hyaluronic Acid Derm Plus 20ml is formulated for consistent, high-quality results in clinical settings. Manufactured under strict GMP standards, it provides superior viscoelasticity and long-lasting hydration, making it ideal for facial and body treatments. Clinics in [Your City/Region] trust our product for its reliability and safety in advanced dermal procedures.
2. Is your sodium hyaluronate raw material derived from animal sources?
No. Our sodium hyaluronate is produced through microbial fermentation, utilizing non-animal-derived raw materials. This minimizes the risk of allergic reactions and ensures high tissue safety for implantable fillers.
3. What is the function of lidocaine in the injectable gel?
Lidocaine (0.3%) is used to minimize discomfort during injection, alleviate local pain, and enhance the patient's treatment experience. Each batch of our product undergoes rigorous quality control to ensure lidocaine concentration and safety in clinical use.
4. How does your cross-linking process preserve hyaluronic acid's natural structure?
Our cross-linking process employs a dual approach: natural entanglement cross-linking of high-molecular-weight HA combined with low-level BDDE chemical modification. This stabilizes the molecular structure of sodium hyaluronate while maintaining its natural biostructure. This balanced outcome delivers superior viscoelasticity, biocompatibility, and water retention during treatments.
5. How can clinics or suppliers collaborate with your factory for OEM or bulk supply?
As a specialized manufacturer and global supplier, we focus on partnering with medical clinics and brand distributors for OEM, private labeling, or bulk supply. We provide comprehensive technical documentation, quality certifications, and global logistics support to ensure safe and efficient delivery.












