Product Overview
Rveiling® PLLA-PEG Microspheres Powder Facial Implant Filler represents advanced PLLA-PEG copolymer technology for biocompatible, biodegradable facial rejuvenation. This single-dose lyophilized powder forms a uniform suspension upon reconstitution with sterile water for injection (SWFI), enabling precise control over injection concentration for cheeks, temples, forehead, and hands.
The product combines PLLA-PEG microspheres with mannitol and sodium hyaluronate, creating a porous sponge-like microstructure for easy storage and transport. Post-injection, it shifts from filling to true tissue regeneration, delivering natural volume maintenance and skin quality improvement.
Key Specifications
Vial Sizes: 220mg/vial and 360mg/vial for flexible dosing.
Composition: PLLA-PEG microspheres, mannitol, sodium hyaluronate.
Sterilization: Irradiation-sterilized for single-use safety.
Shelf Life: 24 months.
Reconstitution: Mix with SWFI to form milky-white suspension; adjust ratio for filling or regenerative needs.
Mechanism of Action
1. How PLLA-PEG Microspheres Work
Biocompatible Scaffold for Collagen Regeneration
PLLA-PEG microspheres act as a temporary biocompatible scaffold, triggering fibroblast activation and collagen synthesis. This controlled response induces natural tissue regeneration, transforming the concept of “filling” into long-term volumetric restoration.
2. Dual Regeneration and Volume Maintenance
Progressive Rejuvenation Process
Post-implantation, PLLA-PEG microspheres are gradually replaced by autologous collagen. This process establishes a durable connective tissue network, improving skin texture, firmness, and elasticity while maintaining facial volume over time.
Product Advantages
1. High Biocompatibility and Safety
Sterilization and Quality Assurance
PLLA-PEG microspheres undergo irradiation sterilization to ensure product safety. The product is single-use only and designed for consistent clinical outcomes.
2. Precision and Control
Adjustable Concentration
By adjusting the dilution ratio during reconstitution, clinicians can control injection concentration to meet diverse tissue filling and regenerative needs.
3. Long-Term Efficacy
Collagen-Based Tissue Reconstruction
The material gradually biodegrades, leaving behind structurally sound, resilient autologous tissue that restores volume and improves skin texture naturally.
Core Technology
1. Advanced Microsphere Carrier System
Optimized Suspension Stability
Rveiling® PLLA-PEG uses highly purified sodium hyaluronate as a carrier, ensuring uniform microsphere distribution and pre-filled vial dosing for clinical consistency.
2. Controlled Biocompatibility
Reduced Inflammation
The carrier system prevents excessive inflammatory response, ensuring safe and predictable clinical outcomes for aesthetic professionals.
Recommended injection sites for PLLA-PEG facial fillers
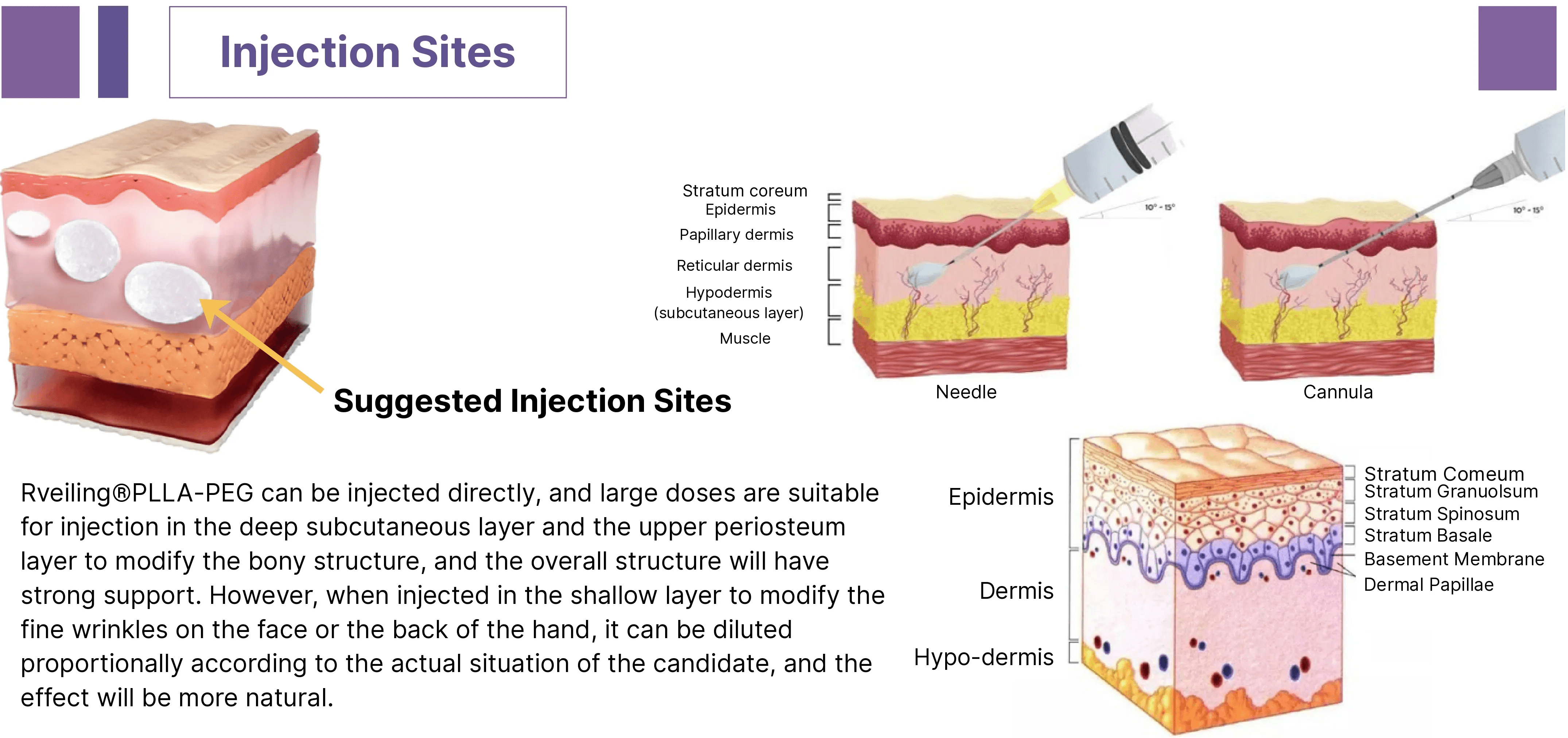
Reveiling® PLLA-PEG Diluted Solution Injection into the Upper Layer of the Periosteum and Deep Dermal Soft Tissue of the Lateral Cheekbone Dosage and Injection Protocol.
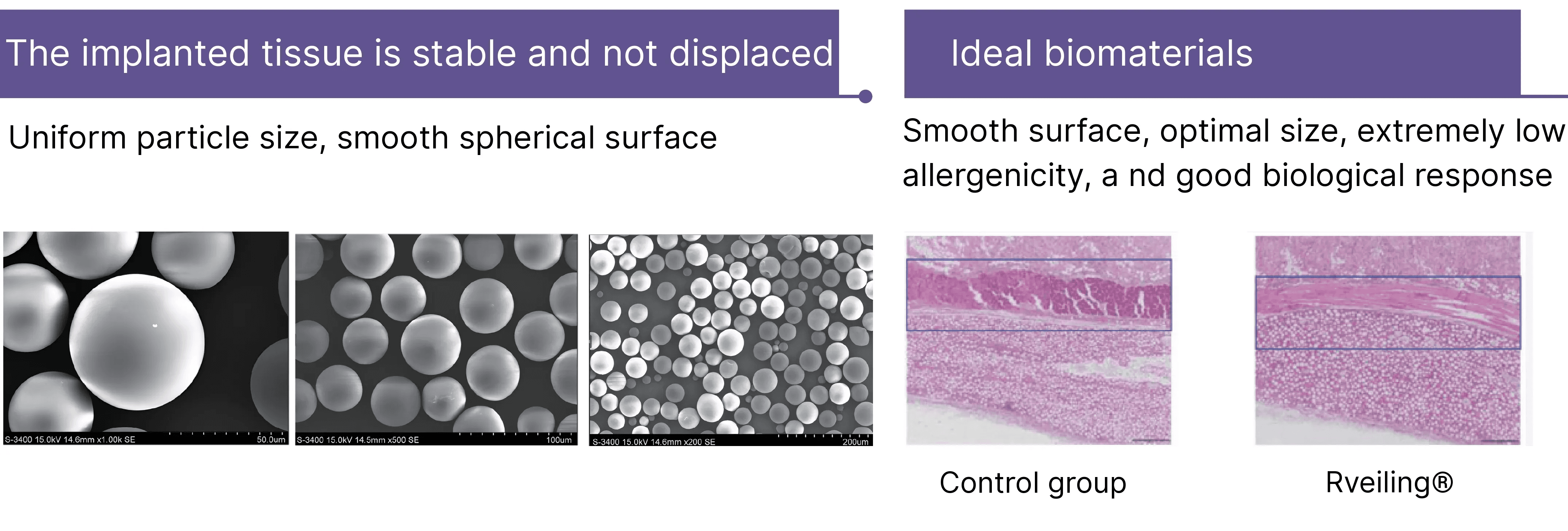
Rveiling® PLLA-PEG microsphere particles remain stable and immobile within tissue, exhibit an extremely low allergenicity rate, and demonstrate favorable biological response. The darker the color, the higher the collagen content.
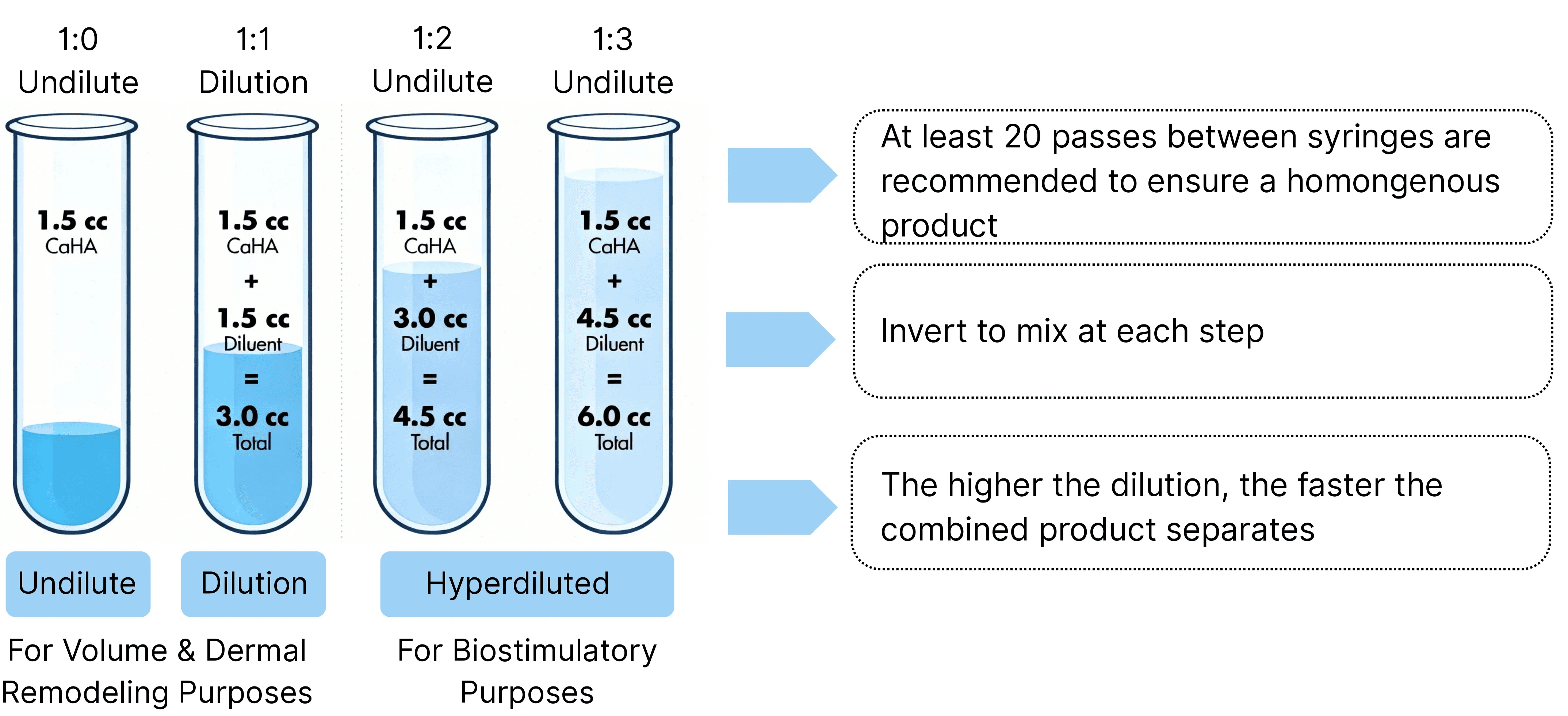
By adjusting the dilution ratio, the clinical injection concentration can be precisely controlled to flexibly accommodate the filling and regenerative needs of different tissue sites.
Facial volume restoration, achieving a fuller appearance
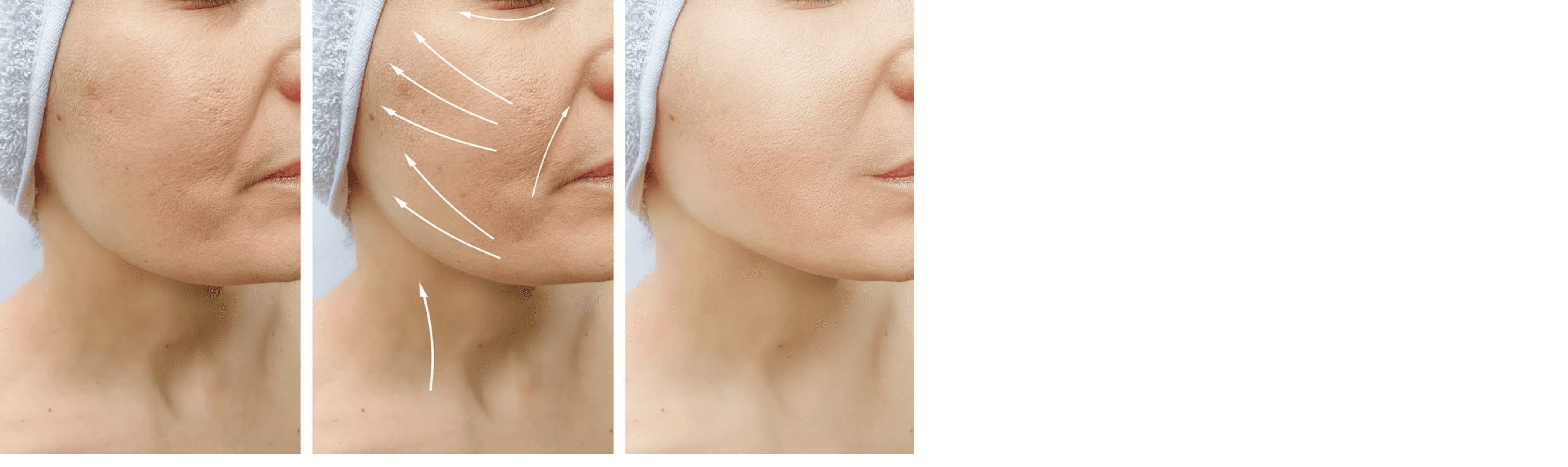
Comparison of Before and After Effects 8 Weeks After Rveiling® PLLA-PEG Diluted Solution Injection Implantation
Key attributes
Storage condition | 2-10°C refrigerated, used up within 24 hours after opening the bottle |
|---|---|
Transport conditions | Transport at room temperature (not exceeding 30°C) should not exceed 1 month, otherwise cold chain transport |
Contraindications |
|
FAQS
1. What are the core competitive advantages of your company's PLLA-PEG lyophilized powder?
Rveiling® PLLA-PEG lyophilized powder for injection originates from its unique material science foundation—PLLA-PEG amphiphilic block copolymer. This intelligent polymer structure achieves breakthrough synergistic effects and clinical performance through precise molecular design.
◆ Intelligent biomimetic structure for optimized tissue compatibility
Within the PLLA-PEG molecule, hydrophobic PLLA segments spontaneously aggregate to form a microsphere core, serving as a supportive scaffold that continuously induces collagen regeneration. Hydrophilic PEG segments extend outward, creating a hydrated shell. This hydrophilic outer layer enhances cell adhesion and tissue compatibility, while the hydrophobic PLLA segments aggregate to form the core and the hydrophilic PEG segments extend outward to form the shell. This facilitates uniform suspension of microspheres within gel particle carriers. This “core-shell” structure mimics the natural extracellular matrix, significantly enhancing the material's hydrophilicity and biocompatibility, creating a favorable microenvironment for cell adhesion and tissue integration.
◆ Mitigating Clinical Risks, Ensuring Natural Efficacy
Benefiting from the exceptional hydrophilicity and dispersibility imparted by PEG segments, the reconstituted suspension of PLLA-PEG exhibits minimal aggregation. Following implantation, it distributes uniformly within tissue spaces. This property physically minimizes the risk of nodule formation due to material agglomeration and significantly reduces unnecessary inflammatory responses. Consequently, it fundamentally prevents the “lumpy” appearance that may occur at the filling site, ensuring smooth and natural therapeutic outcomes.
◆ Rveiling® lyophilized powder not only inherits PLLA's exceptional regenerative capacity but also addresses critical clinical challenges of traditional regenerative materials through PEG's hydrophilic modification. It unifies regenerative efficacy with safety, representing the technological direction of next-generation regenerative materials.
2. What excipients are used in your company's PLLA-PEG lyophilized powder formulation?
The innovation of Rveiling® PLLA-PEG lyophilized powder lies in its novel microsphere carrier system constructed using highly purified sodium hyaluronate. This design enhances product performance across three core dimensions: optimized suspension stability ensures uniform microsphere distribution, pre-filled cartridge technology enables precise dose control, and the carrier's exceptional biocompatibility proactively prevents inflammation. This establishes a robust foundation for safer, more controllable clinical efficacy.
3. Are there specific injection site restrictions or recommendations for your PLLA-PEG lyophilized powder?
Our Rveiling® PLLA-PEG lyophilized powder is suitable for individuals aged 18 and above seeking anti-aging benefits. It enhances skin elasticity, tightens the skin, and improves facial laxity. Diluted at varying ratios, it is applicable for aesthetic treatments targeting the temporal region, cheeks, forehead, hands, skin volume loss, and stimulating endogenous collagen regeneration to achieve long-lasting volume restoration.
4. How long do the effects of your PLLA-PEG lyophilized powder last?
The effects of Rveiling® PLLA-PEG lyophilized powder solutions, which induce sustained collagen regeneration, are long-lasting, typically lasting two years or longer. Results appear gradually over time, yielding a more natural and realistic outcome. Effectiveness and duration depend on the type of defect, injection depth, and individual variations.
5. How long after injecting your PLLA-PEG lyophilized powder product will results be visible? How should expectations be managed and the gradual effect explained to clients?
Results are not immediate, reflecting the product's mechanism of action—a key point B2B partners must clearly communicate to their clients: Rveiling® PLLA-PEG microspheres' ultimate purpose is not “filling depressions,” but reactivating aging skin's self-repair program—first modulating macrophage polarization, then activating fibroblasts, and finally synchronously inhibiting collagen degradation to achieve true “regenerative rejuvenation.”
◆ Initial Injection: Post-injection, the solution causes temporary volume increase. Approximately 1-2 days later, as fluid is absorbed, volume temporarily decreases.
◆ Progressive Effect: True collagen regeneration becomes visible post-treatment. Improvement signs emerge around 4 weeks post-treatment, with optimal results typically achieved after the full treatment course—usually 3 to 5 months.
◆ Expectation Management: We recommend emphasizing the product's “long-lasting, natural, and gradual” improvements in volume and quality rather than “instant changes.” Clear expectation management is key to enhancing client satisfaction and loyalty.
6. Regarding long-term maintenance for PLLA-PEG lyophilized powder products, what follow-up and combination therapy recommendations do you have for B2B partners?
The value of Rveiling® PLLA-PEG lyophilized powder lies in its long-term efficacy. We provide professional guidance for follow-up and combination therapies.
1. Long-term follow-up: Partner institutions should establish long-term client profiles with regular follow-ups every 3 months for 6 to 12 months to assess collagen maintenance and discuss potential consolidation (top-up) injections.
2. Client education: Emphasize that post-procedure massage is a critical component of treatment efficacy. Consistent massage ensures even distribution of microspheres and maximizes collagen regeneration results.













