1. Product Ingredients
High-Purity Formulation
Medical-Grade Ingredients for Professional Use
Rveiling® + Lidocaine Cross-linked Sodium Hyaluronate Gel for Injection is formulated with carefully selected, pharmaceutical-grade ingredients to ensure safety, stability, and consistent clinical performance.
Sodium Hyaluronate: 25 mg/mL
Lidocaine Hydrochloride: 3 mg/mL
Sodium Chloride
Water for Injection (WFI)
Phosphate Buffer System
This high-concentration formulation supports strong tissue volumization, optimal hydration, and reliable performance in cross linked hyaluronic acid dermal filler applications.
2. Mechanism of Action
Advanced Cross-Linked Hyaluronic Acid Technology
High Elasticity and Cohesive Strength
Rveiling® employs high molecular weight hyaluronic acid natural entanglement cross-linking combined with chemical modification, achieving a high cross-linking density and a homogeneous HA gel structure. This advanced cross linked hyaluronic acid technology provides deep tissue support while maintaining flexibility.
Balanced Elastic Modulus and Tissue Integration
Natural, Seamless Aesthetic Results
The optimized balance between elastic modulus (G′) and cohesive force allows the gel to integrate smoothly with surrounding tissue. This results in natural contouring, stable volume retention, and reduced risk of migration, delivering a seamless appearance after injection.
3. Indications and Clinical Applications
Indicated Use
Tissue Volume Augmentation for Adults
Rveiling® + Lidocaine Cross-linked Sodium Hyaluronate Gel for Injection is indicated for individuals 18 years of age and older requiring tissue volume augmentation to improve facial structure and skin appearance.
Injection Layers and Treatment Outcomes
Deep Structural Support and Contour Enhancement
The product is designed for injection into the deep subcutaneous layer or the superficial periosteal layer, providing:
Structural support
Lifting effect
Shape correction
Facial and tissue contour enhancement
It is well suited for professionals seeking a cross linked hyaluronic acid filler for deep tissue support and facial contouring.
4. Core Technology
Patented Cross-Linking Process
Enhanced Stability and Longevity
By combining high molecular weight HA entanglement with chemical cross-linking, this patented technology achieves tighter molecular structure and improved gel stability. The result is enhanced biocompatibility and a prolonged degradation cycle within the body.
Tissue Adhesion and Support
Reliable Performance After Injection
The cross-linked HA gel exhibits excellent tissue adhesion properties, allowing it to remain stable at the injection site while providing consistent support. This ensures predictable, long-lasting volumization and contour retention.
5. Sterilization and Usage
Sterilization Method
High-Standard Medical Sterility
The gel inside the pre-filled syringes is sterilized using high-temperature steam, ensuring medical-grade sterility and safety.
Single-Use Design
Safe and Controlled Application
This product is intended for single-use only, minimizing the risk of contamination and ensuring consistent clinical outcomes.
6. Shelf Life and Storage
Product Stability
36-Month Shelf Life
Rveiling® + Lidocaine Cross-linked Sodium Hyaluronate Gel for Injection has a shelf life of 36 months when stored under recommended conditions, supporting long-term inventory planning for distributors and OEM partners.

Aowita Biotech has obtained EU ISO 13485 quality management system certification, with products compliant with EU CE certification standards. Production cleanrooms meet GMP/Class 100 standards, and quality control adheres to Class III medical device industry standards.
7. Notes
Treatment Area
Rveiling®+ Lidocaine Cross-linked Sodium Hyaluronate Gel for Injection is indicated for use in the deep subcutaneous layer and superficial periosteal layer to provide structural support, lifting, and correction of shape or contour, thereby increasing tissue volume and improving skin appearance. Treatment plans should be individualized based on patient characteristics. A qualified practitioner should assess skin thickness and muscle activity patterns to determine site selection, subcutaneous injection depth, dosage, and other strategic considerations.
Ingredients | Sodium Hyaluronate, Lidocaine Hydrochloride, BDDE, Sodium Chloride, Water for Injection (WFI), Phosphate Buffer System |
Model | Derm Plus/Sub Skin |
Product Specification | 10mL/syringe |
Gel Hardness | Viscoelastic |
Indications | Provides structural support, lifting, and contour correction to improve skin appearance. |
Injection Depth | Deep subcutaneous, supraperiosteal |
Recommended Needle | Blunt-tip cannula |
Treatment Areas | Chest, buttocks, or large-area depressions requiring structural support, lifting, and contour correction. |
The treatment plan should be tailored to individual differences. Professional physicians should select the injection strategy for the middle dermis (fine line repair/moisturizing) or deep dermis (contour lifting/volume replenishment) based on the thickness of the skin and the characteristics of muscle activity.
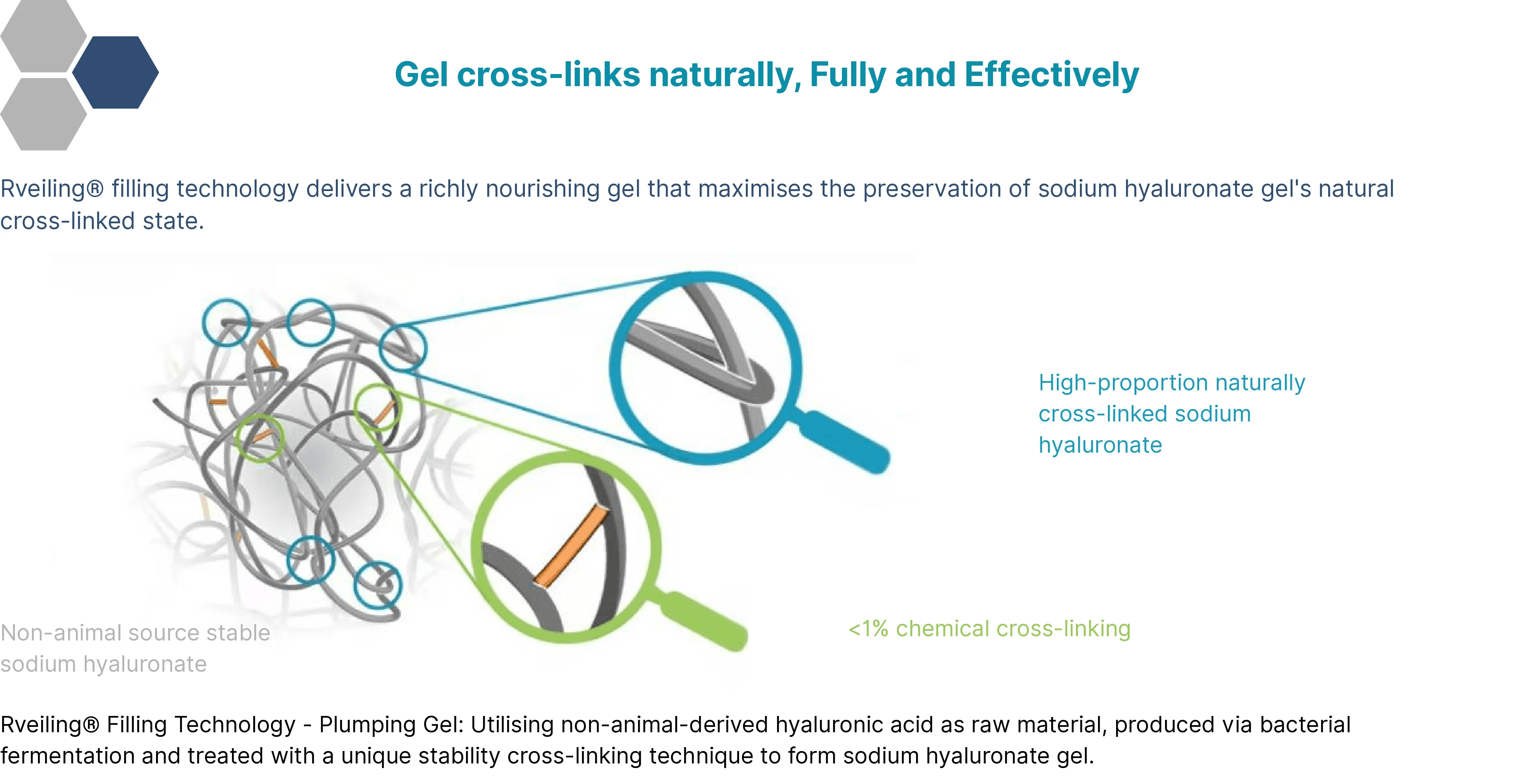
HA Entanglement Crosslinking Technology Gel
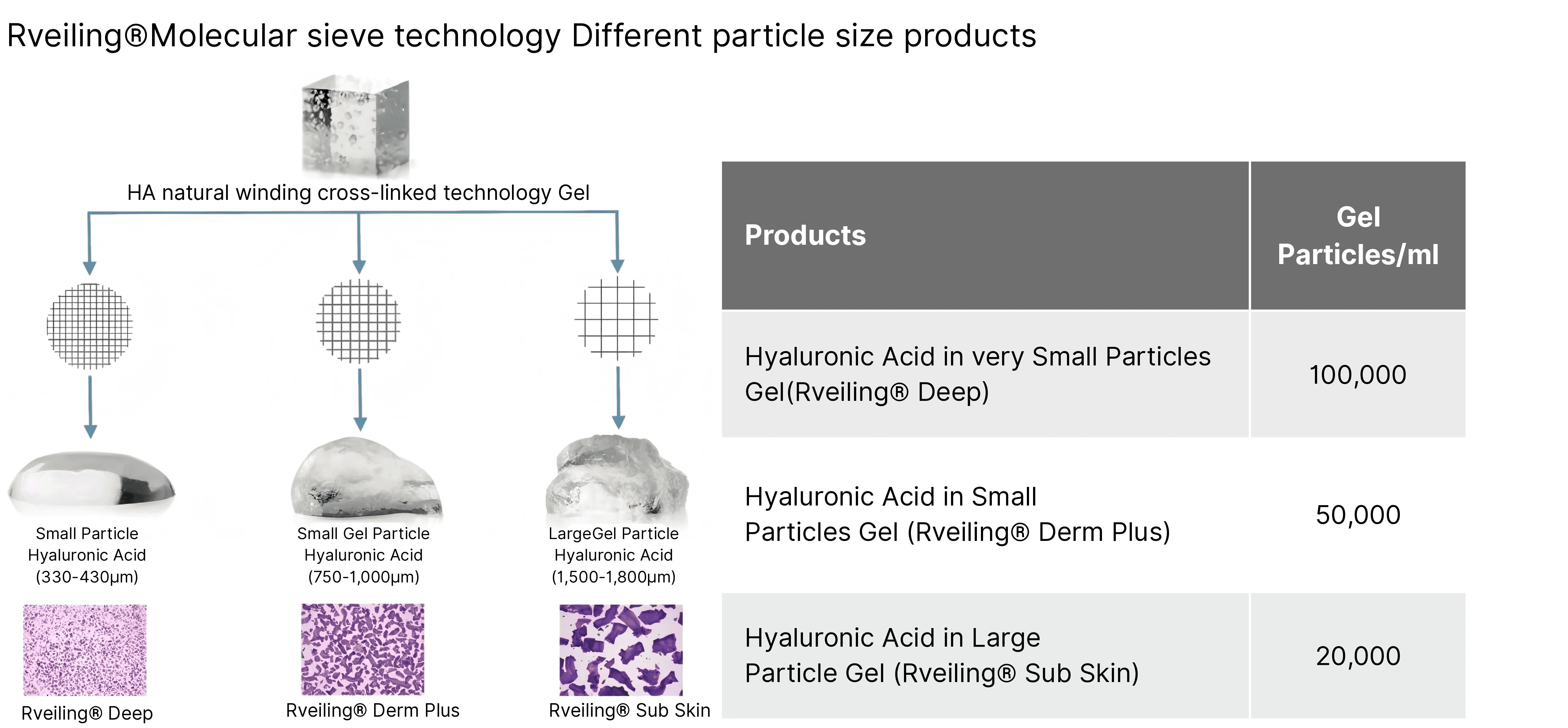
Particle sizes of different Rveiling® products
Revealing@+ Lidocaine Cross-linked Sodium Hyaluronate Gel for Injection begins its preparation with the “natural entanglement” of high-molecular-weight hyaluronic acid (HA) chains, forming an initial three-dimensional network. This is followed by “chemical modification” for precise anchoring and reinforcement, constructing a stable gel molecular structure that combines flexibility with strong support, delivering longer-lasting filling effects.
Key attributes
Ingredients | 20mg/ml Cross-linked sodium hyaluronate |
|---|---|
Gel Hardness | Soft |
Lifting Power | N / A |
Usage | Improve skin appearance |
Injection Levels | Deep dermis |
Product Size | 1mL(One syringe, two needles) |
Suggested Needles | 30 G Sharp needles |
Suggested Treatment Plan | 3 injections, 4 weeks apart |
FAQs
1. Why does cross-linked hyaluronic acid last longer than non-cross-linked hyaluronic acid?
Cross-linked hyaluronic acid possesses a stable three-dimensional structure that resists degradation by natural enzymes in the skin. While non-cross-linked hyaluronic acid breaks down within days to a week, cross-linked hyaluronic acid persists in the body for months or even longer, delivering extended filling and moisturizing effects. Rveiling@+ Lidocaine Cross-linked Sodium Hyaluronate Gel for Injection employs natural HA entanglement cross-linking technology combined with an efficient BDDE-modified cross-linking process. This ensures excellent biocompatibility and long-lasting efficacy while minimizing inflammation.
2. Is your sodium hyaluronate raw material derived from animal sources?
No. Our sodium hyaluronate is produced through microbial fermentation, utilizing non-animal-derived raw materials. This minimizes the risk of allergic reactions and ensures high tissue safety for implanted fillers.
3. What is the function of lidocaine in the injectable gel?
Lidocaine (0.3%) is used to minimize discomfort during injection, alleviate local pain, and enhance the patient's treatment experience. Each batch of our product undergoes rigorous quality control to ensure lidocaine concentration and safety in clinical use.
4. How does your cross-linking process preserve hyaluronic acid's natural structure?
Our cross-linking process employs a dual approach: natural entanglement cross-linking of high-molecular-weight HA combined with low-level BDDE chemical modification. This stabilizes the molecular structure of sodium hyaluronate while maintaining its natural biostructure. This balanced outcome delivers superior viscoelasticity, biocompatibility, and water retention during treatment enhancement.
5. How can clinics or suppliers collaborate with your factory for OEM or bulk supply?
As a specialized manufacturer and global supplier, we focus on partnering with medical clinics and brand distributors for OEM, private labeling, or bulk supply. We provide comprehensive technical documentation, quality certifications, and global logistics support to ensure safe and efficient delivery.












