Product Description
Rveiling® Deoxycholic Acid Lipolysis Injection, with the primary ingredient deoxycholic acid, is a naturally occurring free bile acid in the human body and an endogenous substance produced during digestion. It emulsifies and dissolves dietary fats in the intestines, facilitating their breakdown and absorption by lipase. The Deoxycholic Acid Fat-Reducing Injection is a non-surgical, injectable therapy for localized fat reduction. It primarily works by disrupting fat cell membranes, emulsifying fat into fine particles, and subsequently metabolizing and excreting these particles through the lymphatic system. This achieves targeted fat reduction. Clinically validated for localized fat contouring, it is not a substitute for systemic weight loss. Suitable for stubborn fat deposits in areas such as the double chin, abdomen, and thighs.
Product Name
Rveiling® DCA-Containing Lipolysis Injection Solution
Product Ingredients
Water for Injection, Deoxycholic Acid, Sodium Hyaluronate
Product Specifications
5mL per vial, 5 vials per box
Indications
Chin and other areas with localized fat deposits
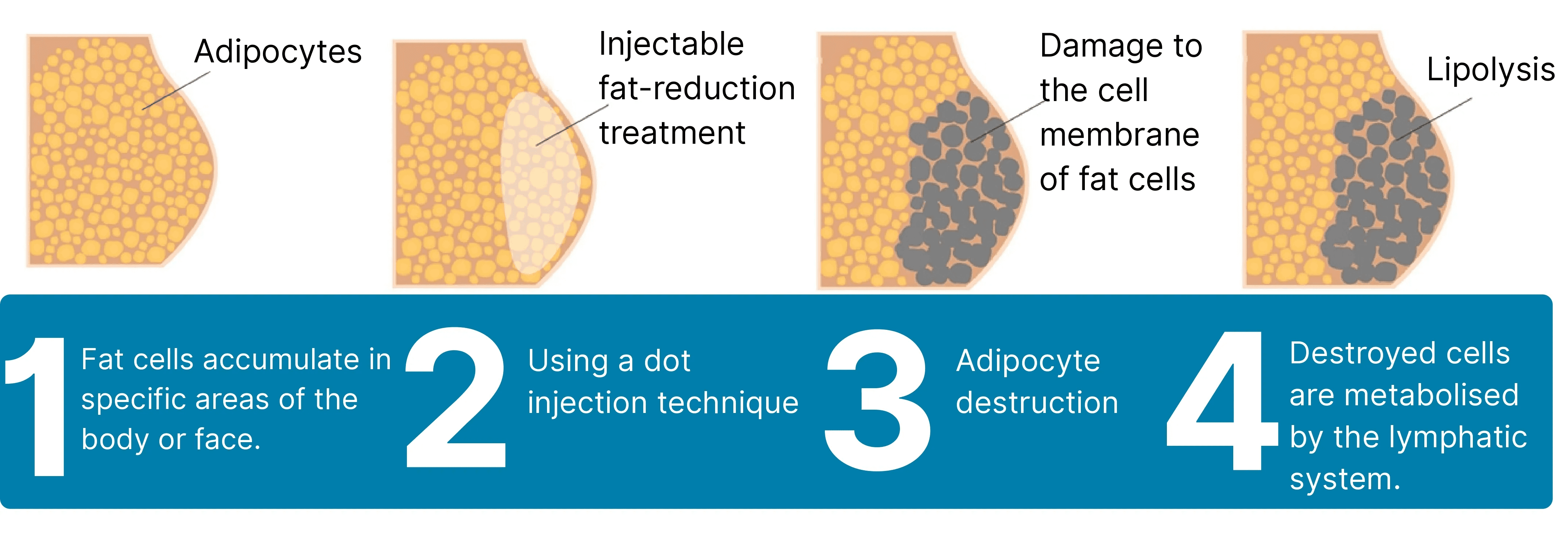
Mechanism of Action
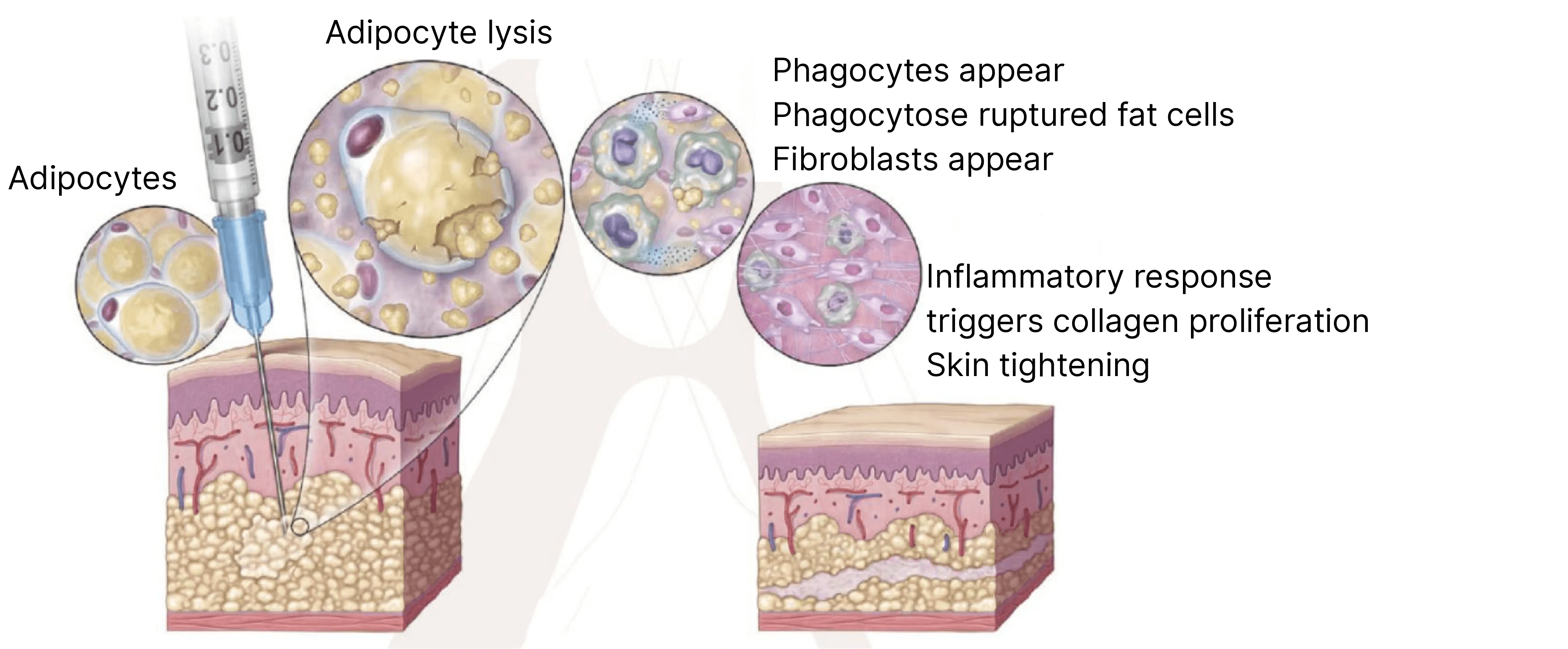
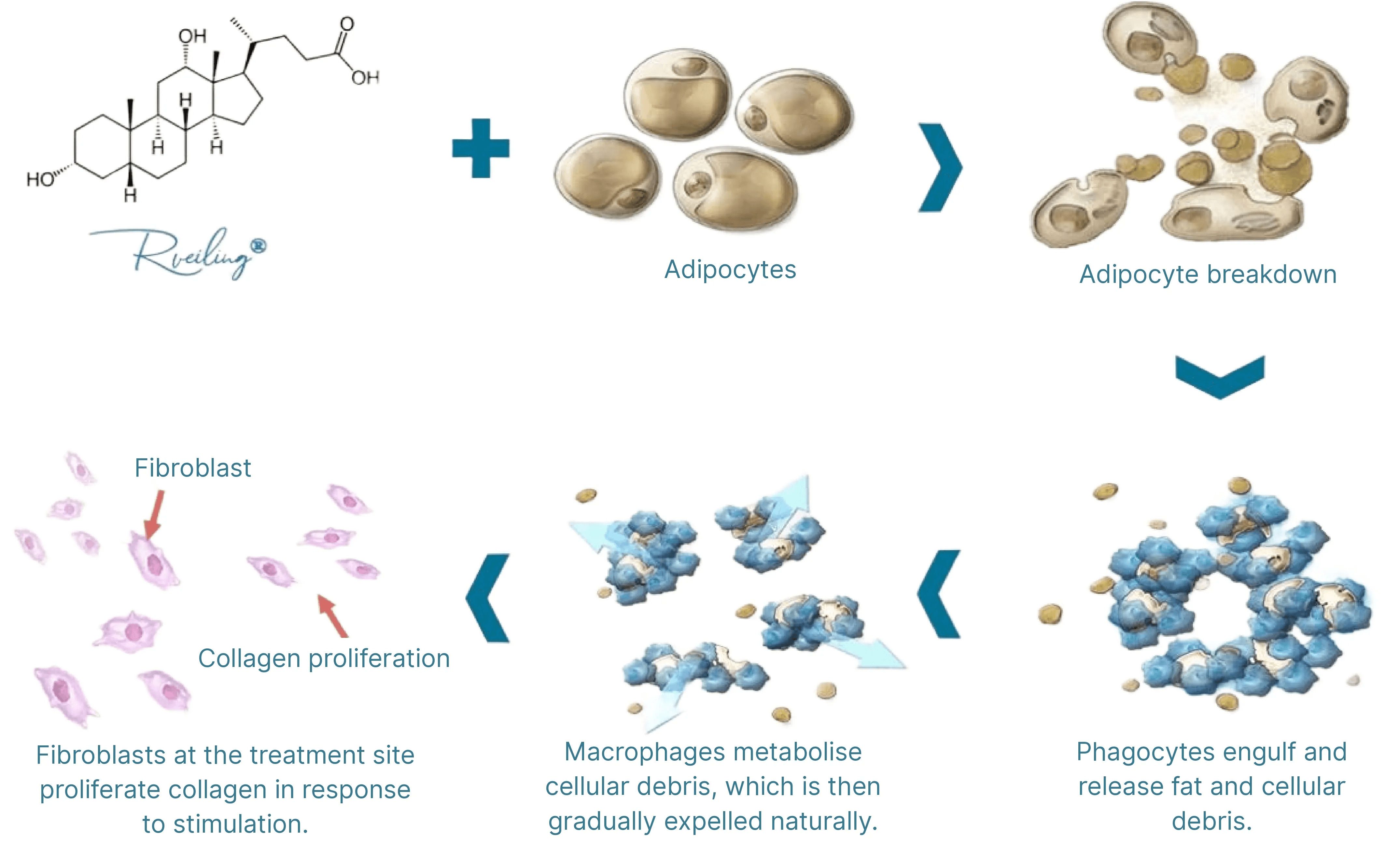
The primary active ingredient, Deoxycholic Acid (DCA), disrupts fat cell membranes, releasing cellular contents. This triggers a localized inflammatory response involving macrophage infiltration and fibroblast aggregation, accelerating fat metabolism. The fat is subsequently eliminated through the lymphatic system as part of normal bodily metabolism, achieving “permanent fat reduction.” This process induces collagen regeneration, promoting skin contraction at the injection site, with results stabilizing approximately 28 days post-treatment. The deoxycholic acid in the injection solution is a synthetic compound, eliminating risks of infection from human or animal-derived pathogens. It fully degrades and exits the body within 24 hours post-injection, restoring endogenous deoxycholic acid levels to pre-injection status, ensuring excellent safety. This ingredient is clinically validated for localized fat contouring but cannot replace systemic weight loss.
Applications
Fat-dissolving injections, facial contouring. Results typically last 3-5 years or longer after 2-4 treatment sessions.
Suitable Candidates
Individuals with localized fat deposits (e.g., double chin, submental area, upper arms, abdomen, thighs).Those with good skin elasticity and no significant laxity.
Shelf Life: 18 months
Storage Conditions: Store at 2–25°C. Do not freeze.
Transportation Conditions: Room temperature (≤30°C) transport must not exceed 1 month; otherwise, cold chain transportation is required.
Note: This product is sterile. Strict adherence to sterile surgical procedures is mandatory during use.
Usage Details: Refer to the package insert.
Key Highlights
Targeted Fat Reduction: Destroys and removes localized fat cells in areas such as the double chin, abdomen, and thighs.
Active Ingredient – Deoxycholic Acid: The core component damages the fat cell membrane, emulsifies fat, and breaks it down into fine particles.
Natural Metabolism: The broken-down fat is metabolized and excreted through the lymphatic system.
Clinically Proven: Demonstrated efficacy and safety for localized fat contouring, though not intended for systemic weight loss.
Minimally Invasive: Offers a non-surgical alternative for facial and body sculpting with minimal downtime.
Scientific Dissolution, Precise Metabolism
● High-Efficiency Emulsification: Targeting the cell membranes of stubborn fat cells, it emulsifies and decomposes them.
● Accelerate Metabolism: Promote the excretion of decomposed fat from the body through the body's natural metabolic system.
● Firming the Skin: The synergistic ingredients help stimulate collagen, assisting the skin in restoring firmness and elasticity while reducing fat.
Key attributes
Deoxycholic Acid | 10mg/ml |
|---|---|
Sodium Hyaluronate | 3mg/ml |
Applicable Crowd | For improving moderate to severe contour prominence or localized fat accumulation causing facial fullness in adults, such as double chin, arms, abdomen, thighs, etc. Suitable for individuals with good skin elasticity and no significant sagging. |
Contraindications | Pregnant women, breastfeeding women, and women during menstruation. Individuals with heart disease, diabetes, or immune system disorders. Those allergic to soy, eggs, or any medication ingredients. |
Storage Condition | 2–25°C. Do not freeze. |
Recommended Treatment Course | 3 to 5 sessions constitute one course, with sessions spaced 15 to 20 days apart (the number of courses may be appropriately increased based on skin condition). |
Injection Depth | Treatment plans should be tailored to individual differences. A professional physician should assess skin thickness and fat characteristics to determine the appropriate injection depth for each area. |
Specific Details | Refer to the package insert for detailed instructions. |
FAQs
1. What is the core mechanism of action for your deoxycholic acid injection?
Deoxycholic acid fat-dissolving injections are a non-surgical, injectable therapy for localized fat reduction. They primarily work by disrupting fat cell membranes, emulsifying fat into tiny particles, and subsequently metabolizing and excreting these particles through the lymphatic system. The deoxycholic acid in the injection is a synthetic substance, eliminating the risk of infection from human or animal-derived pathogens. Doxycholic acid is completely degraded and excreted within 24 hours post-injection, restoring endogenous doxycholic acid levels to pre-injection status with excellent safety. This achieves localized fat reduction. The ingredient is clinically validated for localized fat contouring but cannot replace systemic weight loss.
2. How are adverse reactions managed after lipolysis injection?
Product safety and efficacy are our primary collaboration prerequisites. Due to individual physiological differences, adverse reactions such as swelling, bruising, redness, and pain may occur, typically resolving spontaneously. Post-procedure, avoid high-temperature/humid environments, saunas, strenuous exercise, smoking, and salty foods. Apply intermittent ice packs for 2-3 days, each session lasting 10-15 minutes. Rare allergic reactions, skin necrosis, or nerve damage require immediate medical attention.
3. What are your company's transportation and storage requirements for deoxycholic acid lipolysis injection products?
To ensure product stability and efficacy, we enforce strict regulatory requirements for transportation and storage—a critical quality control aspect in B2B partnerships. Products must be stored between 2°C and 25°C; freezing is strictly prohibited. Partner institutions must maintain standard medical refrigeration equipment. During transit, temperatures should be maintained at room temperature (controlled environment not exceeding 30°C). Extreme temperature fluctuations must be avoided to prevent compromising product potency.
4. What marketing support and training services does your company provide to B2B partners?
As your professional supplier, we are committed to delivering comprehensive B2B support to help you rapidly expand your market presence. Led by senior medical aesthetics experts, we offer specialized training courses covering product mechanisms, injection techniques, and risk management protocols.












